In the fast-paced world of modern manufacturing with expensive materials, many jobs, and very slim margins for errors, maintaining a competitive edge requires more than just skilled management – it demands a seamless integration of key operational elements.
Why Should You Connect Your ERP with Your Manufacturing Quality System?
High QA
One such integration that stands out in its potential to improve manufacturing quality efficiency is the collaboration between Manufacturing Quality Management Systems (QMS) and Enterprise Resource Planning (ERP) systems.
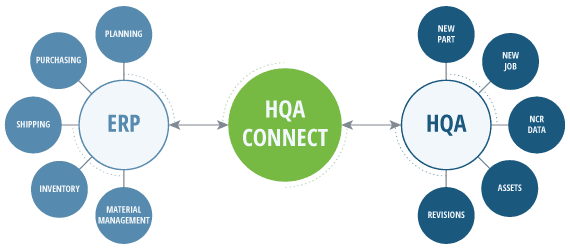
Operational Silos and Quality Compromises
In many manufacturing organizations, the lack of integration between QMS and ERP systems gives rise to operational silos. This division can lead to critical quality lapses, product “lates,” material waste and reduction of overall productivity. To operate an effective business, data and digital content needs to be accurate, consistent, relevant, accessible, and not duplicated. It needs to be entered seamlessly and not manually entered.
Imagine a scenario where the manufacturing quality data is housed in one system while administrative and operational data resides in another with a human in the middle needing to reconcile the data manually. It is a recipe for miscommunication, data errors, and compliance issues. This disjointed scenario not only jeopardizes product quality, cost, and delivery times, but also places unnecessary burdens on compliance and audit efforts.
The Compromise Cost
The effects of such disjointed systems are far-reaching. Audits become a tedious affair, riddled with inefficiencies and potential errors. The fallout? Hefty fines, tarnished reputation, and an erosion of customer trust. Additionally, the inability to quickly identify and address manufacturing quality issues can result in increased product recalls with subsequent financial setbacks. At a time when the market demands agility, these setbacks are not just inconveniences; they’re potential deal-breakers.
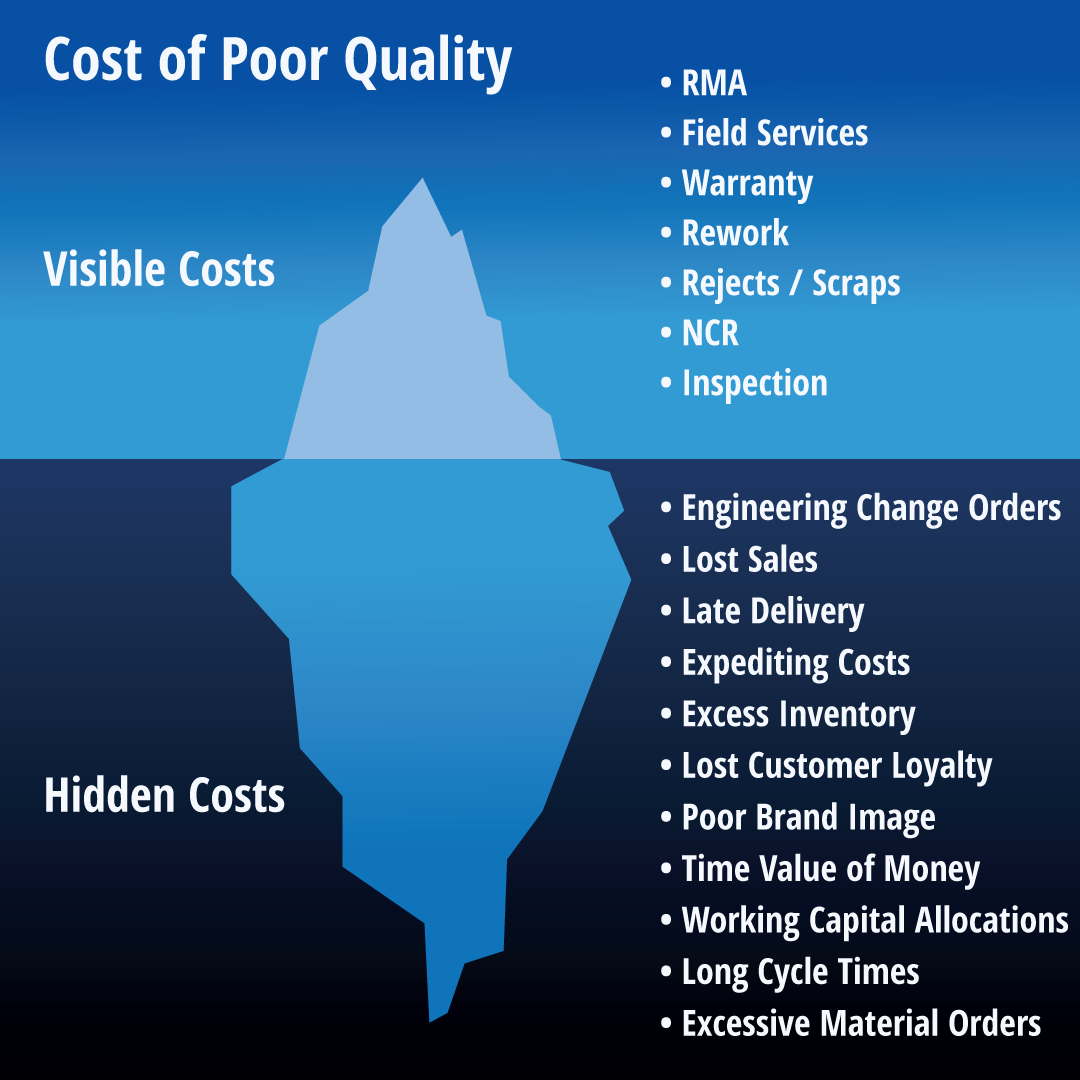
Picture this: a recall due to quality issues that could have been prevented with an integrated Manufacturing QMS and ERP systems. The cost of recalling products, not to mention the damage to brand equity, is a blunt reminder of the price paid for disjointed, non-integrated operational silos.
Unity Through Integration
The remedy to these challenges can be found in the seamless integration of QMS with ERP systems. By fostering collaboration between these two fundamental pillars of business and manufacturing operations, organizations can establish a unified digital thread that diminishes data silos. Quality data then becomes a central part of the entire operational workflow, ensuring that decision-makers have real-time insights into both manufacturing quality and operational metrics.
Let’s think about a scenario where every department, from manufacturing to distribution, is synchronized in real-time, sharing a cohesive data thread. The result? Swift identification and resolution of quality issues streamlined compliance processes, and a proactive approach to risk mitigation. The integration of critical systems doesn’t just bridge the gap – it propels the organization into operational excellence.
Example: Connecting QMS and ERP with HQA Connectors
As we have seen thus far, the integration of ERP and QMS is not just a technological feat; it’s a strategic imperative. The cost of compromise is high, both financially and reputationally. The cost of integration can also be high with the wrong partners. Integrations with custom code or ERP supplied connectors to QMS systems can reach over 6 figures ($100k+) and take months to implement.
Fortunately, using a modern QMS system, like HQA 360, and a modern ERP system with options for open architecture (or APIs) is much easier. For example, creating a new job in HQA 360 software is a swift process, taking only a few minutes. Copying and pasting data from QMS to ERP is relatively common. However, when running hundreds or thousands of jobs each month, that time adds up quickly. As a result, parts are late and working capital goes up.
The HQA ERP Connector removes uncertainties tied to manual job creation. The fear of inadvertently skipping a job, succumbing to typographical errors, or overlooking a critical revision on a new order is now a thing of the past. It seamlessly bridges the gap, performing these intricate tasks automatically between the two systems in the background. The result? More automation, freeing up more time to redirect focus towards tasks of greater significance, and increased productivity!
The Desired Outcome
The painstaking process of data entry is transformed into a real-time automated data sync. No longer is there a need to wrestle with the intricacies of manual input that often is riddled with human errors. Instead, the proven and tested ERP Connector ensures that every job is created accurately, and every piece of information is entered flawlessly.

The manual burden is lifted, allowing quality teams to devote energy to the strategic imperatives that drive businesses forward such as better Reporting and Analytics, and improved utilization of manufacturing machinery.
The HQA ERP Connector is a supported software product, with a lifecycle, enhancements and improvements. It is highly secure and should support compliance needs.
Embrace the power of HQA ERP Connector’s seamless automated integration – where quality and operations converge, and excellence prevails. Break the data silos and improve cost-effectiveness. Elevate your business to a new scalable level. Adopting this software add-on, will positively affect your success.

For more information, visit www.HighQA.com or contact a High QA representative for a no-obligation demo of High QA software, the ultimate manufacturing quality management software.
As you are probably aware by now, Ford Motor Company released their Customer-Specific Requirements for the IATF 16949 standard in January of 2022. The first question many people have is, “If we have a standard for companies to follow, why are there customer-specific requirements on top of the standard? Doesn’t that negate the need for a standard in the first place?” It’s a very valid point and one that’s under continual scrutiny.

However, instead of focusing on the debate on whether there should or should not be additions to a standard, let’s focus on just what some of those changes are – namely the PFMEA requirements.
As anyone who has been in quality more than 13.2 seconds knows, Microsoft Excel is your friend. It’s literally used in place of documents, links, pictures, calculators, and databases. It’s a complete 1-stop-shop for all things in manufacturing requiring data, forms, and some semblance of interconnectedness between them. However, the one huge black eye it does get, is its lack of interconnectedness between it and other systems (without extensive back-end work).

That brings us to the Ford PFMEA requirements. What is a PFMEA? Briefly, PFMEA stands for Process Failure Mode and Effects Analysis. It is a systematic and proactive approach used in various industries to identify potential failure modes in a process and assess their potential effects. The main purpose of PFMEA is to prevent or mitigate potential problems before they occur, thus improving the overall quality and reliability of the process or product.
The PFMEA process involves a team analyzing each step of a process to identify failure modes – ways in which the process could potentially fail to produce an acceptable part. For each failure mode, the team then assesses its severity (impact on the process), occurrence (likelihood of the failure mode occurring), and detection (likelihood of the failure mode being detected). The outcome of a PFMEA is a prioritized list of potential failure modes, ranked by their Risk Priority Number (RPN), which is calculated by multiplying the severity, occurrence, and detection ratings. This rating allows the team to focus on high-risk areas and develop appropriate actions or controls to prevent the identified potential failures.
From a documentation standpoint, a common practice with a PFMEA is to take the documentation of a part that is similar to the part you are compiling the submission package for and ‘copy and paste’ it over to the new submission package and change the necessary information (failure modes, occurrences, etc.) that are relevant for the new part. This is an inherently error-prone process with items missed or omitted and not entirely reviewed properly or comprehensively. Therefore, when the final PPAP submission package is created, the PFMEA being a vital component of the PPAP, it is rejected, and the supplier has to fix and resubmit.

This is the issue that Ford is correctly trying to prevent.
The new Ford PFMEA standard requires the information associated with the Processes identified on the PFMEA to be generated from a controlled repository of data – ideally, but not specifically, from a database. An Excel-generated PFMEA will not be allowed or approved by FoMoCo.
The High QA PQP module takes this challenge head-on and conforms to the new Ford-specific requirements on top of the IATF 16949 standard by utilizing a database-driven Process Library within the Inspection Manager application. This Process Library contains all necessary “master” information for the Processes at your facility, including Severity, Occurrence, and Detection information and auto-calculated RPN values. This “master” information is used to auto-generate the PFMEA document within High QA.
What does the PFMEA generation process look like? Simply this:
- Balloon the print (High QA automates this too!)
- Identify the characteristics linked to each manufacturing operation
- Link the Process to the Operation (simple pull-down menu)
- Generate the PFMEA
PFMEA Generation will become an automated, consistent, and controlled process that can be done in a few minutes instead of the manual, error-prone Excel method of old.
Contact High QA or visit us at major industry events to find out more about PPAP submission generation using High QA PQP!

For more information, visit www.HighQA.com or contact a High QA representative for a no-obligation demo of High QA software, the ultimate manufacturing quality management software.
As industries progress and advance, so do the standards that govern their operations. One such standard is AS9102. While primarily developed for the aviation, space, and defense industry, this standard is also used in other manufacturing industries where a standardized First Article Inspection (FAI) process is needed.

This standard plays a crucial role in manufacturing and quality with guidelines for planning, manufacturing, and verifying that the processes can produce parts and assemblies that meet the engineering requirements.
In June 2023, the SAE Aerospace Standard (AS) announced an upgrade of the AS9102 standard from Rev. B to Rev. C, bringing about changes that manufacturers must now follow. One of the more important updates is the requirement for more thorough tracking which adds an extra layer of complexity and accountability to the FAI process. This new provision aims to establish complete traceability, ensuring that each product is consistently manufactured at the highest quality.
For some manufacturers, the new requirements introduced in AS9102 Rev. C might appear overwhelming and potentially tedious. The additional required information could demand extra time and resources. However, these measures have been implemented to enhance quality control and traceability, ensuring that every product meets the highest standards of excellence.
What's New in AS9102 Rev. C?
The transition from AS9102 Rev. B to Rev. C has brought about some enhancements that manufacturers need to embrace:
Increased Tracking Requirements
AS9102 Rev. C raises the bar by mandating the tracking of certain items. This level of traceability ensures accountability and transparency throughout the quality process. Manually fulfilling these standards could take a long time and be error-prone, opening the door to expensive mistakes. However, with High QA, tracking and documenting this crucial data becomes a breeze, streamlining your FAI process while effortlessly maintaining compliance.

Improved Documentation
Compliance with AS9102 Rev. C demands comprehensive documentation of the FAI process. With new forms and documentation requirements, manufacturers face the challenge of managing vast amounts of data accurately. High QA, as a robust quality automation platform, simplifies this task by organizing and centralizing all relevant data, allowing your team to generate fully compliant AS9102 Rev. C documents with ease. Say goodbye to the hassle of sifting through paperwork and embrace the efficiency of digital documentation.
Enhanced Alignment with AS9100
AS9102 Rev. C is now more closely aligned with AS9100, the quality management system standard for the aerospace industry. This alignment is designed to facilitate integration and ensure that the FAI process seamlessly complements your broader quality management efforts. High QA not only helps you meet AS9102 Rev. C requirements but also assists in establishing a comprehensive and coordinated quality management system, elevating the overall performance of manufacturing and quality processes.
Form Changes in AS9102 Rev. C
Rather than covering all the standard revisions, let’s look at the changes directly affecting Forms 1, 2, and 3.
For a comprehensive understanding of the changes, you can get a copy of the AS9102 standard through SAE International.

Below is a list of form changes from Revision B and Revision C. The most significant changes between the two versions have been highlighted. Even though some of the changes are minor, it is still important to be aware of them so that you can update FAI processes and train the quality team.
High QA has dynamic fields on many forms. These fields ae usually highlighted with yellow. New data can be entered into the field or existing data can be modified. Some of the forms include an “Add” button to dynamically add more rows for certain fields in boxes. Also, some companies don’t allow for blank fields. High QA includes a parameter, “Fill Empty Field With”, that will input the specified text such as “N/A” into empty fields as applicable.
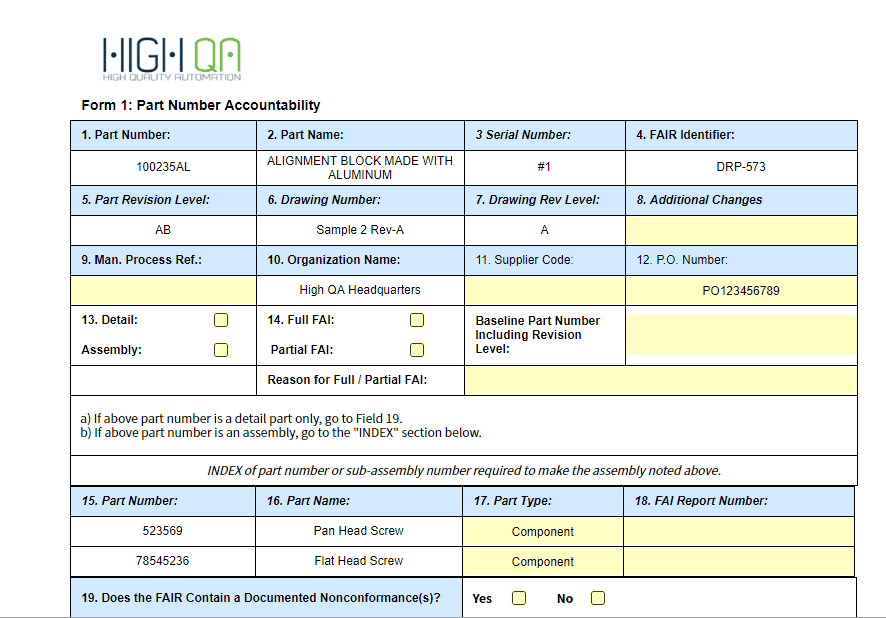
Form 1
- Field 4 – FAIR Identifier was Field 4 – FAIR Number
- Field 13 – Detail and Assembly was Field 13 – Detail Part and Assembly FAI
- Field 14 – Reason for Full / Partial FAI was Reason for Partial FAI
- Field 17 – Part Type was Field 17 – Part Serial Number
- Field 19 – Does FAIR Contain a Documented Nonconformance(s)? has been added (Sign offs and respective Date fields were renumbered)
- FAI Complete and FAIR Not Complete were removed
- Field 20 – FAIR Verified By was Field 19 – Signature
- Field 22 – FAIR Reviewed/Approved By was Field 21 – Reviewed By
- Field 23 – Date was Field 22 – Date
- Field 24 – Customer Approval and Field 25 – Date are now conditionally required
- Field 26 – Comments was added
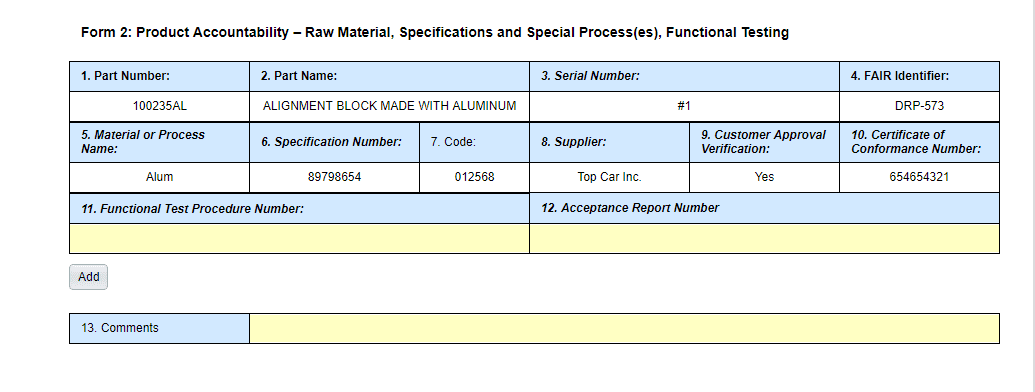
Form 2
- Field 4 – FAIR Identifier was Field 4 – FAIR Number
- Field 14 – Signature and Field 15 – Date have been removed
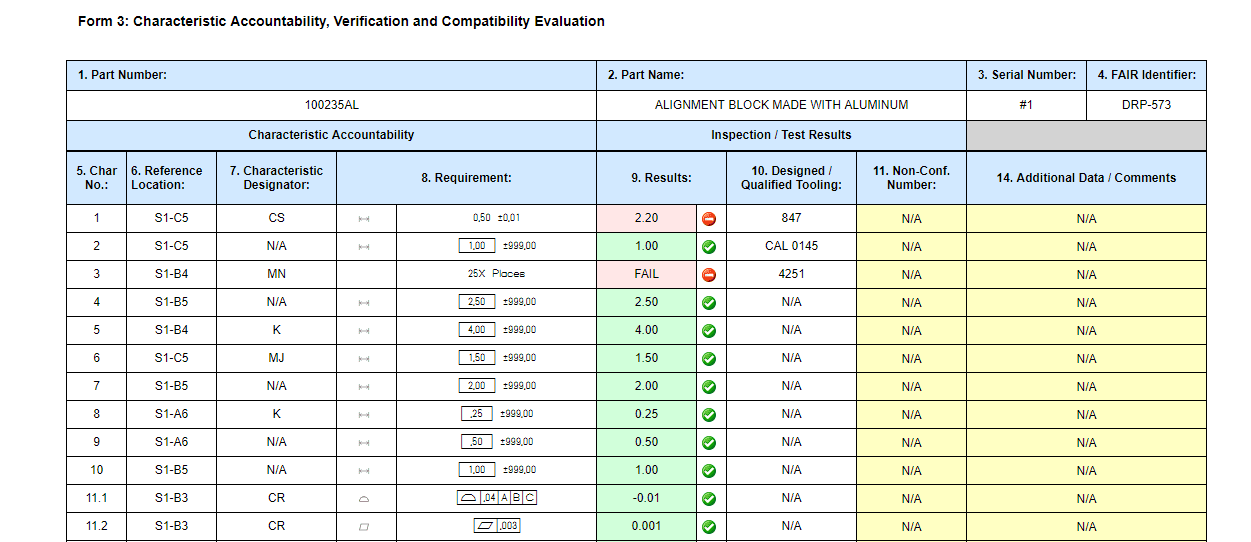
Form 3
- Field 4 – FAIR Identifier was Field 4 – FAIR Number
- Field 12 – Signature and Field 13 – Date have been removed
How High QA Can Help
High QA emerges as the ultimate solution to navigate the complexities of AS9102 Rev. C. This cutting-edge software has been specifically engineered to streamline manufacturing quality automation and optimize the first article inspection process. With High QA, compliance with AS9102 Rev. C is a breeze, offering manufacturers several invaluable advantages.
Comprehensive Data Tracking
High QA makes sure that each measurement is carefully recorded with all the pertinent data. This end-to-end traceability helps develop an accountability culture and is essential to producing an AS9102 Rev. C document that is fully compliant.
Time-Efficient Documentation
High QA automates the data collection and documentation process, significantly reducing the time required for creating comprehensive FAI reports. This not only accelerates the manufacturing quality process but also frees up valuable resources for other critical tasks.
Real-Time Data Insights
High QA enables data-driven decision-making by providing real-time insights into the quality management processes. With the aid of the data analytics and reporting tools that High QA provides, problem areas are pinpointed, and quality processes are streamlined.
Enhanced Productivity
By automating data collection, analysis, and reporting, High QA empowers employees to focus on core quality tasks, leading to improved productivity and overall efficiency within the organization.
Scalability
High QA grows with your needs, making it a future-proof investment. Whether a small business or a large enterprise, High QA adapts to the size and complexity. As a business expands or as industry standards evolve further, High QA is designed to accommodate these changes.
Staying Ahead
In the face of ever-evolving industry standards, staying ahead can be challenging. With AS9102 Rev. C imposing more stringent requirements, companies need tools that make compliance not only achievable but efficient. Embrace the future of manufacturing quality automation with High QA – your reliable partner in achieving AS9102 Rev. C compliance effortlessly.

Investing in High QA has benefits beyond just complying with the updated specifications of AS9102 Rev. C; it also provides cutting-edge technology to keep your company productive, competitive, and dedicated to producing high-quality goods.
Get the tools you need to produce high-quality goods, uphold standards that are industry-leading, and promote success in your company. Embrace the power of High QA now to reach the full potential of your quality process, increase productivity, reduce errors, and meet customer expectations.
Learn more about FAI at /fai/ and request a demo at /schedule-a-demo/
Request a web-meeting demo by requesting a demo now or visiting us at major industry events.

For more information, visit www.HighQA.com or contact a High QA representative for a no-obligation demo of High QA software, the ultimate manufacturing quality management software.
Show Your Boss the Benefits of Quality Management Software
High QA
In today’s competitive manufacturing environment, maintaining first-rate quality is of vital importance for organizations across various manufacturing industries. Whether it’s general manufacturing, aerospace, automotive, medical, or any other industry, maintaining detailed quality control processes is necessary to meet customer expectations and industry standards.
However, managing ballooning drawings, First Article Inspection (FAI), Production Part Approval Process (PPAP) and other quality processes can be complex and time-consuming without the right tools. That’s where Quality Management Software (QMS) like High QA 360 comes into play, offering a comprehensive solution to automate, streamline and optimize the critical manufacturing quality processes.
Before approaching your boss, it is crucial to understand the benefits of implementing High QA 360. Let’s look at the key benefits of investing in the High QA Manufacturing Quality Suite for ballooning drawings, FAI, PPAP, and other quality processes and how those can convince management to make the purchase.

Benefits of Quality Management Software for Manufacturing
Enhanced Efficiency and Time Savings
Quality Management Software automates and digitizes the entire ballooning drawing process, FAI, and PPAP documentation, reducing the need for manual effort and paperwork. By eliminating tedious tasks such as data entry with the possibility of cut and paste errors, document routing, and version control, employees can focus on more value-added activities.
High QA 360 provides a centralized repository for ballooning drawings, making it easier to access and share information across departments, suppliers, and customers. This streamlined approach results in significant time savings, accelerating production timelines and reducing overall lead times.
Many High QA customers have told us they have up to an 80% time reduction in part planning and decrease report production time up to 50%.
Improved Accuracy and Compliance
Manual processes are prone to human error, which can have severe consequences in quality control. QMS software reduces the risk of errors by automating data capture, calculations, and validations. It ensures that ballooning drawings are accurate, FAIs are comprehensive, and PPAP documentation meets the necessary standards and requirements. By maintaining consistency and adherence to regulatory guidelines, manufacturers can implement High QA 360 to avoid costly errors, rework, and non-compliance issues.
Enhanced Collaboration and Communication
QMS software provides powerful data analysis capabilities that help manufacturers gain valuable insights. Management can see a more complete picture of quality measurements, trends, and performance indicators by utilizing powerful reporting options and customizable dashboards. This data-driven strategy helps identify areas for improvement, optimize processes, and make informed decisions to drive continuous improvements projects.
Data Analysis and Reporting
In complex manufacturing or supply chain environments, effective collaboration and communication are vital for successful quality control. Quality Management Software provides a centralized platform where teams can collaborate on ballooning drawings, FAIs, PPAP and other quality documents in real-time. It allows everyone to work together, share feedback, and resolve issues promptly. High QA 360 provides improved visibility and transparency so that managers can monitor progress, track approvals, and ensure all necessary stakeholders are involved, enhancing cross-functional collaboration.
One High QA prospect lamented about having reams and reams of data but none of it was useful until High QA software was implemented. Now, with a few simple clicks, reports can be generated from data in the centralized database keeping it up to date and consistent.
Cost Reduction and ROI
While the initial investment in Quality Management Software may seem significant, the long-term benefits outweigh the costs. By automating and streamlining processes, reducing errors, and improving efficiency, QMS helps organizations minimize waste, rework, and non-conformance expenses. Automating the generation of documents reduces costs associated with manual data entry and administrative tasks. Ultimately, the return on investment (ROI) from High QA 360 implementation is realized through improved product quality, enhanced customer satisfaction, and a competitive advantage in the market.
A large manufacturing company has seen an average of 72% savings on quality planning and reporting processes.
High QA Investment
Investing in Quality Management Software is a strategic decision that can revolutionize how organizations manage ballooning drawings, FAI, PPAP and manufacturing quality processes. The benefits of enhanced efficiency, improved accuracy, streamlined collaboration, data analysis, and cost reduction make a compelling case for adopting QMS.
By leveraging the power of technology to optimize quality control processes, organizations can elevate their product quality, ensure compliance, and achieve customer satisfaction. Convincing management to purchase High QA 360 is not just an investment in software, but an investment in the overall success and growth of the organization.
Learn how the High QA 360 manufacturing quality suite can help your company achieve higher profitability and growth.
Request a web-meeting demo by requesting a demo now or visiting us at major industry events.

For more information, visit www.HighQA.com or contact a High QA representative for a no-obligation demo of High QA software, the ultimate manufacturing quality management software.
You Are Closer to AS13100 Than You Think
High QA
February 1, 2023
Standards are intended to help organizations establish and maintain effective quality management systems and processes. They help ensure manufacturing sites are able to consistently provide products that meet customer and regulatory requirements.
AS13100 is the latest aerospace standard to be released. This standard creates a common set of supplemental requirements to improve understanding, efficiency, and performance.
The primary intent of this new standard is to improve overall product quality, by focusing on the key systems and processes currently deterring consistent aerospace engine product quality.
AS13100 will be a required supplement to AS9100 and AS9145 that focuses on customer specific requirements for aircraft engine OEMs.
Along with the industries key standards of AS9100 and AS9145, the recent released AS13100 is designed to prevent defects and waste. Integrating this new standard is essential in developing, maintaining and improving a more effective and efficient QMS.

Do You Have To Follow The AS13100 Standard?
Given that AS13100 is a voluntary standard, manufacturers are not obligated to abide by it. However, many businesses in the aerospace and defense sectors decide to put the standard into practice in order to show their dedication to quality and to live up to consumer and regulatory expectations.
Some organizations may be required to comply with the standard as a condition of doing business with aerospace and defense customers. For example, a supplier of parts or services to a major aerospace or defense company may be required to have a quality management system that is certified to AS13100 or a similar standard.

It may still be beneficial to implement the standard even if you are not required to do so. It can raise customer satisfaction levels, improve product quality, and boost a company’s overall effectiveness.
What are the benefits of following the AS13100 standard?
Implementing the AS13100 standard can provide several benefits to an organization, such as:
- Improved Quality: By implementing the standard, you can establish and maintain an effective quality management system that helps ensure products meet customer and regulatory requirements.
- Improved Customer Satisfaction: You are better able to understand customer requirements and meet or exceed those requirements, which leads to increased customer satisfaction.
- Improved Efficiency: Identifying and eliminating inefficiencies in quality processes often leads to cost savings and improved productivity.
- Improved Competitive Advantage: Demonstrating your commitment to quality to customers and regulators can lead to a competitive advantage in the marketplace.

- Improved Compliance: The standard helps you comply with applicable laws, regulations, and industry standards, which can reduce the risk of costly non-compliance issues and part recalls.
- Improved Risk Management: By identifying, assessing and managing risks, you can reduce the likelihood of incidents and accidents, and mitigate the impact of those that may occur.
What Is In AS13100?
Many of the needed documents and reports in the AS13100 standard can be found in the PPAP requirements.
PPAP includes 18 required documents and reports:
Design Records
Engineering Changes
Engineering Approval
DFMEA
Process Flow Diagram
PFMEA
Control Plan
MSA and Gage R&R
Dimensional Results (FAI)
Material / Performance Records
Process Capability Study
Qualified Laboratory Reports
Appearance Approval Report
Sample Production Parts
Master Sample
Checking Aids
Customer Requirement
Part Submission Warrant
The most common PPAP submission package is Level 3. A level 3 PPAP contains 16 of the 18 elements with only “master sample” and “checking aids” being omitted. The AS13100 standard incorporates these 16 PPAP requirements.
Why AS13100 can be a challenge?
All of the required PPAP reports have to be generated. That is a great deal of paperwork just for one single part. And some of the documents are more time consuming and challenging than others.
- Process Flow Diagram
- Control Plan
- PFMEA
- MSA (Gage R&R)
- Process Capability Study
- Part Submission Warrant
Just generating these elements takes a large amount of time and effort, when there are disjointed processes, software and systems.
Often times, prints are ballooned in one software, measured in another, with data compiled in another software or put on a PDF that has to be manually transferred to an FAI. SPC is calculated in another software, gages are tracked manually or in a standalone software while Gage R&R is calculated and compiled in yet another software.
On top of all of that, the Process Flow Diagram, the Control Plan, and PFMEA are typically manually generated in Excel.
There are so many different forms, spreadsheets, documents and lists that need to be maintained, tracked and coordinated.

Many companies hire a specific person or a group of people just to do the paperwork for a submission warrant.
High QA Helps Simplify AS13100 Documentation
High QA software helps you automate your manufacturing quality process and streamline all the reports and documents required by regulatory standards and customer requirements.
High QA software provides the tools to digitize the quality process and increase collaboration throughout your organization.
From the time you acquire the information from your customer, the collaborative digital lifecycle begins. It usually starts by receiving a 2D drawing or 3D model from your customer.
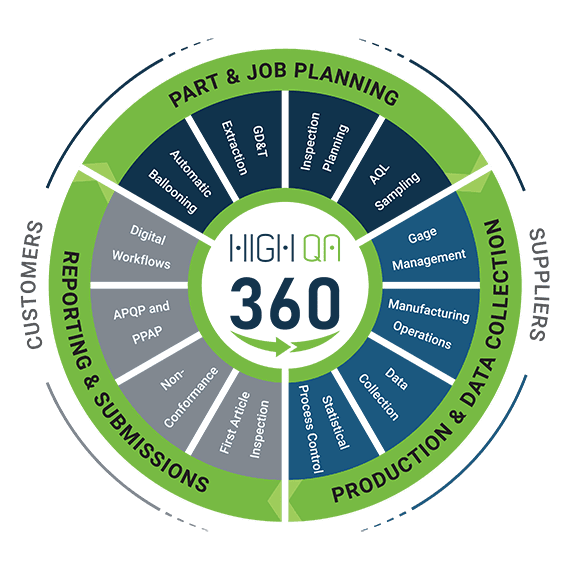
Then you can begin the planning phase with one-click to balloon the drawing, extract the information from it (including the GD&T), create inspection plans, assign measurement methods and sampling rates.
On the production side, you can manage all the gages in your facility, collect all the inspection data, and provide SPC data, including notification for out of tolerance conditions.
Once the plan is made and the data is collected, this information is used to generate the submission package. No more spreadsheets with multiple tabs and pages, everything is in a database. Everything that is done is live and real-time in the database.
Now you can simplify and automate your part submissions and become compliant with the AS13100 standard or any other industry standard. That makes you more efficient, more profitable and successful.
If your company wants to achieve higher profitability and growth, let us show you how integrated manufacturing quality software works by requesting a demo now or visiting us at major industry events.

For more information, visit www.HighQA.com or contact a High QA representative for a no-obligation demo of High QA software, the ultimate manufacturing quality management software.
The 11 C’s of PFMEA in Manufacturing Quality
High QA
October 25, 2022
“If you can’t measure it, you can’t improve it,” is a statement often used by business management thinker Peter Drucker. He means that you don’t know whether or not a process is successful unless it is defined, measured and tracked. For quality and manufacturing risks, that is known as PFMEA.
Process Failure Mode and Effects Analysis (PFMEA) plays a major role in quality-centric manufacturers. Many times, a PFMEA is needed to meet compliance standards or it is part of your customer’s requirements. Even if a PFMEA is not required, many manufacturers still use it to meet and improve quality initiatives.
”If you can’t measure it, you can’t improve it.
Peter Drucker
What is PFMEA?
The Process Failure Mode and Effect Analysis (PFMEA) is a risk assessment method used to analyze and evaluate potential failure modes of processes. It helps to establish the impact of a failure and identify and prioritize the action items needed to correct and alleviate the risk.

It is a living document that is usually initiated prior to process of production and maintained through the lifecycle of the product. PFMEA is a manufacturing quality diagnostics tool that identifies and drives corrective action to prevent or decrease the possibility of defects being delivered to the customer.
When Do You Perform a PFMEA?
It is a good practice to identify risks for each process step as early as possible. The main goal is to identify risk prior to beginning the manufacturing process. Lessening of an identified risk prior to first article inspection (FAI) or Production Part Approval Process (PPAP) will validate the expectation of process performance.
Risks can be introduced to a process when
- There is a new technology or new process introduced
- There is a modification to a current process
- There is a change in location
The C's of Powerful PFMEA
When considering software to help with PFMEAs, it is helpful to consider the 11 powerful C’s of PFMEA.
Control
- Control over your entire manufacturing, quality and submission processes.
Continuous Improvement
- Continuous improvement of manufacturing with a focus on risk management and action monitoring.
Collaboration
- Collaborate with engineering, manufacturing, inspection and quality teams to consider all potential failure modes.
Comply
- Comply with ISO, AIAG and VDA standards and requirements.
Capture
- Capture engineering, manufacturing and inspection best practices, procedures and knowledge.
Centralized
- Centralized PFMEA processes, flow charts, control plans, inspection procedures and workflow charts in a single database.
Comprehensive
- Comprehensive digital workflows enable control over who creates, modifies, approves and signs.
Consistent
- Consistent failure mode descriptions across all documents, parts, submissions and supplier submissions.
Cut Down Stress
- Cut down stress during audits with up-to-date and easy-to-find documents from initial plans to PPAP files.
Clarity
- Clarity in all aspects of quality and PFMEA processes.
Complete
- Complete start-to-finish process that incorporates control plans, process flow diagrams and PFMEA worksheets.
Choosing PFMEA Software
The powerful C’s of PFMEA identify areas where a PFMEA software can aid in identifying potential failures associated with the manufacturing process such as human error, equipment malfunctions and production bottlenecks improving your manufacturing quality management process.
You can show evidence that potential failure modes and risks have been addressed at the manufacturing process level during PPAP.
High QA software enables you to perform manufacturing quality diagnostics of your quality process. You can be assured that your PFMEAs are organized and complete.

All parts of your process control management – P-Diagram, Process Flow Diagram, PFMEA Worksheet and Control Plan – are readily available and integrated for consistency ensuring that all elements are included.
Learn how you can make your manufacturing quality process completely integrated and seamless with High QA software. Contact High QA to learn about our quality management and manufacturing software and schedule a free one-on-one demo.
For more information, visit www.HighQA.com or contact a High QA representative for a no-obligation demo of High QA software, the ultimate manufacturing quality management software.
Quality 4.0 and the New Era of Manufacturing Quality
High QA
The manufacturing world is continuously evolving, with new concepts and technologies coming to light each day. These evolutions enhance the product and service delivery in the manufacturing sector.
Industry 4.0 represents the dawn of digital transformation. The impact of digital data, analytics, connectivity, scalability, and collaboration are the drivers empowering the fourth industrial revolution and forming Quality 4.0 strategies.
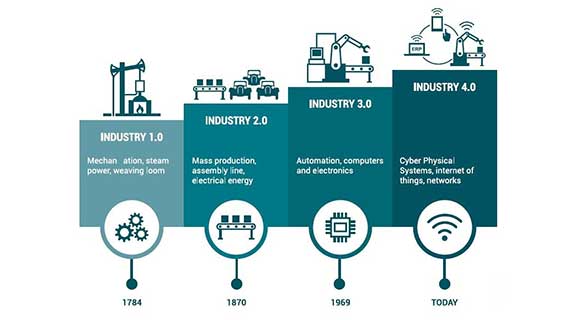
As an industry player, you want to stay on top of all the innovations and advancements to produce quality products and cut through the competition.
One of the recent concepts in manufacturing is Quality 4.0. The primary aim of this model is to help organizations achieve manufacturing quality excellence.
What Is Quality 4.0?
Quality 4.0 is simply the digital transformation of quality management in the manufacturing sector. The concept is all about aligning the quality management software and practice with the emerging capabilities of Industry 4.0.
The primary driver of this quality-driven revolution is the shift to digital working in the manufacturing sector. It uses Industry 4.0 technologies, integration, and digitalization to steer greater robustness, productivity and efficiency.

Quality 4.0 is about getting ready for the future by equipping your organization with connected, advanced, and purpose-built tools. These tools and technologies will make your quality processes effective while increasing visibility. As a result, you’ll quickly achieve your organizational goals.
The Aspects of Quality 4.0
Quality 4.0 is an extension of Industry 4.0 that is based on multiple components. Let’s examine the principles of quality assurance to help you understand this concept better.
Data
Data has always propelled improvement in the quality sphere. However, some organizations lag in data collection, analysis, and decision-making. A primary element of the Quality 4.0 is the swift and effective data collection from multiple sources to empower agile and informed decision-making through quality control system management.
Connectivity
Connectivity in Quality 4.0 is the tie between engineering, manufacturing and quality teams. Quality 4.0 connects them together allowing teamwork, real-time or near real-time data collection and continuous improvement.
Collaboration
Quality 4.0 incorporates a blockchain that includes all shareholders; manufacturers, suppliers, customers, quality systems manager, and management.
Analytics
Quality 4.0 enhances the application of machine intelligence or big data for data analysis.
Quality Management System (QMS)
The implementation of a QMS, such as High QA, will automate processes, provide a single source of data and supply user with all the template needed to create important documentation.
Scalability
Quality 4.0 offers manufacturing firms unlimited, connected, and cloud-based scalability.
Compliance
The concept automates compliance activities through various tools and techniques. It delivers connected, automated, and configured requirement for all manufacturing standards such as AS9102 and ISO standards.
Leadership Buy-in
The quality control software establishes quality KPI and extends to executive ownership.
Competency
It provides quality management solutions that prioritize training and shared experiences to enhance experiences.
Culture
Establishes a quality culture by empowering people with transparency, responsibility, and connected access.
Why Manufacturers Need Quality 4.0
There are various benefits of Quality 4.0 to manufacturers in various sectors. Quality 4.0 aligns the best quality management practices with the digital environment.
A quality control manager who has invested in the concept will achieve significant value chain improvements across service or operational efficiency, company culture, and customer satisfaction. And it’s not just about the technology but also the people and processes that use technology and quality system software.
Quality 4.0 does not replace the traditional quality management processes. Instead, it builds and improves the practices to enhance operational excellence within an organization.

Additionally, Quality 4.0 enhances an organization’s quality performance through top management support for quality. Therefore, organizations should incorporate various quality management system software to steer operational excellence.
Implementing Quality 4.0
Synonymous with smart manufacturing, Quality 4.0 focuses on transforming manufacturing quality processes from paper-based methods to automated digital processes. This quality concept in the manufacturing sector is aimed at helping organizations achieve operational excellence. It has various components: data, connectivity, analytics, compliance, and competency, among others.
Manufacturers need Quality 4.0 and quality management software solutions to enhance operational excellence.
As experts in manufacturing quality and supply chain success, High QA enables manufacturing companies to implement Quality 4.0 initiatives with one integrated software solution to manage the quality process internally and across the supply chain.

Quality 4.0 has never been easier to implement. Make your quality management process completely integrated and seamless with High QA software. Contact High QA to learn about our quality management and manufacturing software and schedule a free one-on-one demo.
For more information, visit www.HighQA.com or contact a High QA representative for a no-obligation demo of Inspection Manager, the ultimate manufacturing quality management software.
5 Tips To Consider Before Starting Your High QA Software Journey
High QA
June 22, 2022
You have made a great step in the right direction for your manufacturing quality management by purchasing High QA software. And now you need to integrate it into your work environment.
But implementing software is a daunting task that often cause businesses to struggle.
It is hard to know how detailed your rollout plan should be without adding unnecessary steps that could delay your timeline.
That is why you need to have a software implementation plan in place. Proper implementation will maximize the value of High QA software so that you can quickly take advantage of the process and efficiency improvements it provides.
5 Tips for High QA Software Implementation
To make your plan easier, here are 5 tips you should address before you start your High QA software journey.
1. Appoint a High QA Software Champion
Implementing a new software platform requires coordination, communication, and follow through – this is the role of the champion inside your organization. Your champion should be empowered to lead the High QA software internally. They will also receive specialized training from the High QA Professional Services team to help them be successful.
2. Define Your Goals & Timeline/Phases
What do you want to achieve using High QA software? Be specific, such as “I want to reduce inspection planner creation time by 50% and go to paperless data collection and reporting.” Specific goals create accountability for users and provide a tangible cause for effectively adopting the new software. Having specific goals and an idea of your timeline expectations, will help the High QA team match you to the right products and focus on your desired outcomes.
3. Plan Training & Implementation Time
When you purchase High QA software, the High QA customer success team will start coordinating your installation, training, and implementation. These steps will involve people from your organization, so budgeting their time for the project is a critical step. Based on the products your purchase, the High QA team will advise your training and implementation roadmap so you can plan appropriately. It is crucial that everyone on your team is aligned with each other. Consider hosting workshops to ensure alignment and keep everyone informed on updates and changes to the software implement plan.
4. Prepare Your IT Team
Let your internal or external IT provider know that you are purchasing a new software platform and will need their assistance with the installation process. The installation is the first step, so good coordination helps get the installation completed quickly so you can start using High QA software.
5. Be Open to Evolving Your Quality Processes
You are implementing High QA software for a reason and the High QA team wants you to achieve those goals. During implementation, your current quality process will be discussed and suggestions, best on best practices, may be advised. Evolving internal processes is always a customer decision but be open to change if it helps you achieve your desired outcomes.
Keep It Positive
No matter how great and intuitive High QA software is, implementation doesn’t equal adoption. You must put strategies in place to garner user acceptance and adoption of the new system.
Without positive engagement around the software, you risk the adoption falling flat. You could fall behind on your implementation timeline or see employees not using High QA as planned.
After Implementing High QA Software
You are probably elated to get running with High QA software, but you need to master walking with it first. It might take some time to get up to full speed, and that’s fine—just continue working better with the software each day.
Training is a central pillar in continuous improvement.

Consider that not every user in the group will be able to dedicate the same amount of time to training, and as such, High QA can provide stakeholders with different training options.
Try out different types of training to employ throughout your software implementation plan:
Lunch and Learns
Introduce workshop-style training in an informal setup. This is best used for collaborative learning and brainstorming sessions where users share success stories and work together to solve issues.
Peer-to-Peer Meetings
Ask your power users and champions to provide one-on-one coaching to share how the tool has increased their productivity.
Self-Training
Encourage users to take advantage of the High QA Academy, webinars, reference guides and other training to further their knowledge and understanding of the High QA software. Contact High QA Support to register for the High QA Academy.
We are Here to Help You Succeed
Using the five steps above will help ensure you get the most value out of your new software.
High QA provides industry-leading professional services and support. We are here to help you get the most out of your High QA manufacturing quality management software investment.

The High QA Resources Portal is an online comprehensive knowledge base. The extensive library of articles and videos will help you get up to speed, sharpen your skills, or explore new topics. If you’re in need of support at any time, High QA’s sophisticated ticketing system makes submitting support requests easy and pain-free.
At High QA, we are here to help your business be more successful. When you’re ready to see what we can do for your manufacturing and quality management, contact us today and let our professionals help you get the high-quality support your business needs.

Explore our website and discover what High QA can do for you and your suppliers. We are just a click away to answer questions or provide a demo of our software.
For more information, visit www.HighQA.com or contact a High QA representative for a free no-obligation demo of Inspection Manager, the ultimate manufacturing quality management software.
Ensure Quality, Safety and Compliance in the Aerospace Industry with Quality Management Software
High QA
June 6, 2022
Quality management is vital in the aerospace industry to meet specified standards. Delivery of high-quality products is crucial to customer satisfaction and if quality falters there can be severe safety consequences. Organizations that do not adhere to the standards face multiple quality challenges, and most do not survive in the industry.
A recent report shows that the Boeing 787 aircraft is still plagued with quality issues and had several omissions in its quality documentation submitted towards the end of April 2022 leading to business interruptions.

The organization halted deliveries since it needed to work through inspections and repairs to streamline the operations. This clearly shows that quality engineers, managers, decision-makers, parts inspectors, and other leaders need to ensure there is quality, safety and compliance in the aerospace industry.
Fortunately, today, technology continues to enhance operations within the aerospace industry. Now there is quality management technology to meet quality requirements and ensure compliance with aerospace industry standards.
What Is Quality Management in Aerospace?
Quality in the aerospace industry is a standard set of guidelines that ensure organizations within the aerospace industry meet requirements and continue to improve their processes. Organizations comply with the set standards to achieve quality policies and objectives.
A quality management system (QMS) helps the aerospace industry achieve compliance, leading to streamlined operations. For instance, High QA software enhances planning, inspecting, and reporting. This promotes quality management and compliance in the aerospace industry both internally and throughout the supply chain.

The International Aerospace Quality Group (IAQG) produces the standards that every organization within the industry must abide by. However, organizations face challenges meeting the requirements because they lack the right tools. Quality management software can simplify the process and meet the quality management system requirements.
What Is the Role of Quality Management in the Aerospace Industry?
Quality management plays a crucial role in the aerospace industry. It helps ensure that equipment, systems, and processes meet the set standards to manufacture quality products.
Full Traceability
Traceability helps the aerospace industry find the product information and understand the history of its parts. Delays can lead to interruptions of the production processes. Having quality management software helps the industry ensure effective reporting and accountability for each part during each manufacturing stage. It helps you know whenever something goes wrong, determine the exact point and ensure compliance.
Automation and Streamlining Audits
Performing internal and external audits helps operators within the aerospace industry to comply with the set standards. Using a QMS automates and streamlines the quality audit process. Operators can use the software to assign audit teams, create auditing schedules, monitor performance, manage online and offline audits, generate analytical reports, and track preventive and corrective actions. This, in return, reduces operation costs, increases employee efficiency, and eliminates duplicate systems.
Supply Chain Quality Management
Effective management of the supply chain boosts communication and collaboration. Using High QA 360 software eliminates the interpretation of errors and minimizes the manual process. The software also helps in effective planning, inspecting and reporting, managing assets, and forecasting. The manufacturing management can use the forecasts to determine risks and mitigate them before they occur.
Increased Safety and Consistency
Safety is vital in the aerospace industry. As an engineer, operator, or manager within the aerospace industry, you not only have to focus on complying with the standards, but also need to ensure safety, consistency and quality of the products. This is crucial because the aerospace sector’s end products, consisting of spaceships, defense crafts, and airplanes, carry many passengers each day. Passengers must be protected at all costs, and therefore quality management is essential.
Reduced Costs
The manufacturing and production process often comes at a high cost and a lack of quality processes and documentation can lead to major financial issues like Boeing has experienced with the quality of the 787 aircraft. Implementing a quality process can be cost-effective with quality management software. Quality manufacturing software like High QA 360 minimizes the time for First Article Inspection (FAI) and PPAP documentation, making it cost-effective in the long run. While you may spend money purchasing the software, you experience a higher return over the long term.
Enhance Quality in Aerospace Industry with High QA Quality Management Software
Quality management can be more effective and streamlined with automated solutions. Make your quality management process completely integrated and seamless with High QA 360 software. Contact High QA to learn about our quality management and manufacturing software and schedule a free one-on-one demo.

For more information, visit www.HighQA.com or contact a High QA representative for a free no-obligation demo of Inspection Manager, the ultimate manufacturing quality management software.
Quality Assurance (QA) and Quality Control (QC) in Manufacturing: Differences You Need to Know
High QA
April 28, 2022
An organization must be aware of the differences and definitions of Quality Control (QC) and Quality Assurance (QA).
Both are essential components of an organization’s quality management strategy, and the success of quality processes are contingent upon all stakeholders, including management and all employees comprehending the distinctions.
An effective quality process can significantly contribute to the success of a project. Still, when they are misunderstood, they are likely to be useless in ensuring that the manufactured part is delivered on time, built within the team’s budget, and meets the customer’s needs.
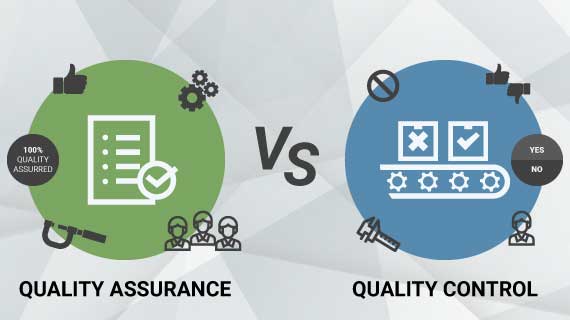
What is Quality Control (QC)?
Quality Control, usually abbreviated as QC, is concerned with identifying defects. QC verifies that the part is manufactured within the design standards and ensures that deliverables adhere to stated quality requirements.
Quality Control is a reactionary process that is based on detection. It is used to identify flaws in a manufactured part.
What is Quality Assurance (QA)?
Quality Assurance is abbreviated as QA and is concerned with fault prevention. Quality Assurance guarantees that the projects’ approaches, techniques, methodologies, and processes are implemented correctly.
Quality assurance activities check and verify that the processes for managing and creating deliverables are being followed and are functioning correctly.
QA is a proactive procedure that is geared toward prevention. It identifies process weaknesses and areas for improvements.

Quality Assurance (QA) or Quality Control (QC)
To fully comprehend the distinctions between quality assurance and quality control, you must first understand how the two processes interact to improve your organization’s quality and eliminate corrective actions.
1. Dedicated Personnel in QC vs Entire Team in QA
The entire staff participates in quality assurance operations. QC on the other hand has a select department or group of individuals responsible for finding flaws. QA activities are the responsibility of every participant in the manufacturing facility.
QA operations include defining processes, team training, and educated tool selection. Auditing is also a part of quality assurance.
2. Process-oriented in QA vs Product-oriented in QC
Quality assurance is a process-oriented discipline that focuses on preventing quality problems. QC focuses on discovering quality concerns in manufactured products that may affect consumer satisfaction. Actions vs results is another way to think about this dichotomy. QA is concerned with the processes, whereas QC is concerned with the finished product.
3. QA is Proactive while QC is Reactive
Quality assurance that works is proactive. Its goal is to use processes to prevent problems from occurring in the first place. QC is a reactive process that seeks to find faults in product quality after a part is made.
Every time you follow the quality process, the result should be a safe, effective product. Testing items to ensure that they fulfill safety and efficacy criteria is known as quality control. If you discover quality issues during QC testing, you should take immediate action to prevent a dangerous product from being transported and disseminated.
In an ideal world, QC concerns would also prompt a QA audit. Non-conforming test findings should trigger a corrective and preventative action study to uncover the root cause of quality issues and improve manufacturing and quality processes to avoid recurrence.
4. Quality Assurance is based on Process, while QC concentrates on Verification
QA efforts produce a plan for developing high-quality goods. Some aspects of quality assurance are standardized in the ISO 9000. In addition to these standards, manufacturers should further develop and employ their own internal standardization practices.
QC entails checking products after they’ve been manufactured and before they’re distributed and ensuring their safety and efficacy.
QC is usually the part of an organization whose responsibilities include following standard operating procedures for product testing. Quality control personnel follow standard operating procedures (SOPs) and document their findings using standardized product testing and process validation techniques.
The Benefits of Quality Assurance
Quality assurance provides multiple benefits to manufacturers who make QA a priority.
Increased Cost Savings
As a proactive component of quality management, good QA leads to the prevention of quality issues. There are fewer scrap parts and material, returned products and recalls.
Increased Production Efficiencies
With fewer defective products, manufacturers can allot resources such as time and money to additional projects. It takes fewer resources to produce quality goods if processes are in place that support quality manufacturing.

Increased Customer Satisfaction
When manufacturers employ effective quality assurance techniques, customers receive better products on faster timelines and with greater levels of consistency. With a lower likelihood of receiving a defective product, customers can also see benefits further down the line for additional projects with greater personalization and innovation. This equates to greater customer retention and increased revenue.
Using High QA for Quality Assurance
Maintaining quality is a constant challenge for manufacturers. Using information from quality control, manufacturers can implement corrective quality assurance processes to make parts on specifications, on time and on budget.
High QA provides the ideal solution for both quality control and quality assurance. As a single source of truth for quality within your organization all authorized employees can securely access QA and QC processes and data and use that information to generate all the necessary FAI, PPAP and submission package documents.

Our manufacturing quality management solution (QMS) software will help you save 75% of your time. Take your manufacturing from good-to-great with an all-in-one quality software solution.
Explore our website and discover what High QA can do for you and your suppliers. We are just a click away to answer questions or provide a demo of our software.
For more information, visit www.HighQA.com or contact a High QA representative for a free no-obligation demo of Inspection Manager, the ultimate manufacturing quality management software.