Inspection Software Evolving to All-In-One QMS Solutions for Practical Data Management
Data-driven manufacturing is here.
Inspection Software Evolving to All-In-One QMS Solutions for Practical Data Management
Data-driven manufacturing is here.

An automated, centralized, integrated approach to inspection and measurement data management can
significantly reduce errors, improve data security, and provide consistent product quality.
Those of us on the march towards Quality 4.0 in our manufacturing companies are grappling with the challenge of producing high quality parts with a minimal investment of time and resources into new inspection methodologies. Additionally, there’s the overwhelming data management aspect. How do we know that it’s “good” data? Where is the data stored? How can we best present it to our customers, quality system auditors, and internal staff? Those are just a few of the driving questions and issues surrounding data and automating the quality function. Until recently, there were way too many individual pieces of software crunching data from machines, devices, instruments, and gages and storing it in independent, disconnected folders housed in a myriad of places, including desk drawers! Here’s the good news: software solutions are available now that truly streamline inspection and measurement data and even go beyond that functionality.
At the risk of this sounding too elementary, like a Quality 101 workshop, let’s review why an understanding of manufacturing process data and managing it is important. For new technicians who are coming into industry daily, these fundamentals are important to grasp. It’s also helpful for us seasoned veterans to get a refresher now and then: The ultimate benefit for a company to understand and house the minutia of its processes is improved profitability. Wider profit margins stem from machining and operational efficiencies that come about from continuous improvement tweaks. We learn what needs to be fixed from the data as we learn how the process is trending over time. Making components better, cheaper, and faster leads to improved deliveries and satisfied customers who likely come back to us again and again.
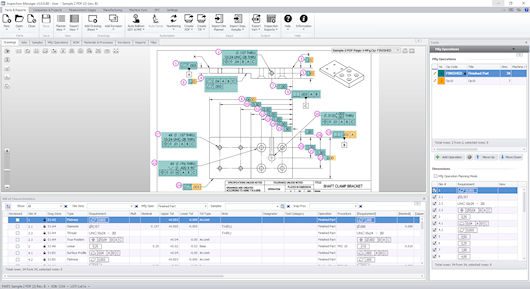
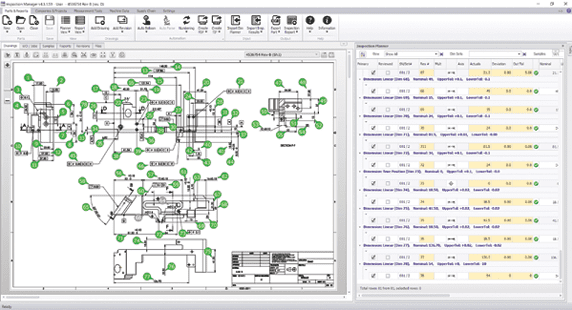
Screen shot displays automatic ballooning and GD&T extraction on a scanned 2D drawing. This task,
done automatically, saves sometimes days of time. It is also helpful assurance that the initial inspection and
measurement data collected at the start of the work order process is reliable and accurate.
Organizing data and making it easily accessible are the keys to getting the best use out of it. Initially, that can be a challenge for many manufacturers. We frequently find that data is stored in various folders, as “silos” of information that aren’t linked in any way, either to each other or to a centralized system or network. So ideally then, we need a comprehensive manufacturing quality management system (QMS) that features centralized data storage where manufacturing results are automatically posted as measurements are taken, compare these results against the inspection plan instantly, and provide alerts when process adjustments need to be made in real-time. Today’s comprehensive QMS software technology gives us the best chance of delivering useful information from the beginning of the work order. One-click automation software now allows us to capture all the relevant measurement and GD&T data from the 2D drawing or 3D part model. Some customers report that this benefit alone saves them days, not hours, of time. As part of this step, these systems then generate a complete bill of characteristics for the part, in-process and final inspection plans, SPC settings, and identifies the gages and equipment required for inspection.
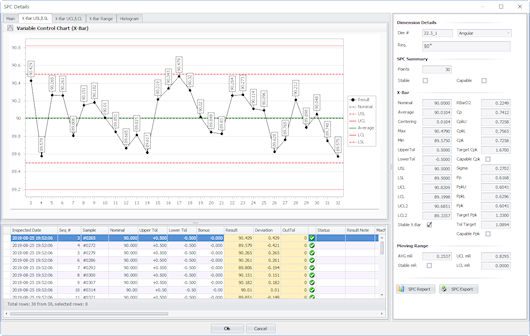
Comprehensive, all-in-one QMS software technologies feature centralized data storage where manufacturing
SPC results are automatically posted as measurements are taken, compare these results against the
inspection plan instantly, and provide alerts when process adjustments need to be made in real-time.
For those of you well on the road to a systems approach to manufacturing, you may ask whether this level of QMS software can integrate with your ERP system. Yes, it can. Quality 4.0 merges with Industry 4.0 easily. In fact, the Quality 4.0 pillar of the higher level plant-wide goal, is imperative to the success of Industry 4.0. Manufacturers first began digitizing aspects of their business and production operations decades ago, and that effort often resulted in “silos” of information using non-connected software programs. I can see the relief on the faces of our customers who have made the shift to an all-in-one platform as they start realizing the benefits. They like the immediate ease of having one central system with which to interact. Many of our customers have the system dashboard visible and accessible at many places around the factory – in production cells on computer stations and using small, portable devices; on big screens mounted on walls to see the status at a glance; or on computer kiosks accessible at convenient locations. Inspection results are imported automatically into the system from CMMs, VMMs, arms, and Bluetooth or wireless devices or manually via fully integrated shopfloor tablet-based apps running on standard devices.
The latest generations of comprehensive QMS technology gets all contributors in the
manufacturing process on the same page with part dimensions, tolerances, and
data reporting which maximizes process efficiency.
Another important aspect of instituting a comprehensive manufacturing QMS is its ability to easily generate reports for quality auditors and customers – including the stringent reporting requirements in the aerospace and medical industries. Report types include industry-standard, FAI, PPAP, SPC, NCR, ISO; and industry-specific such as AS9100/EN9100, AS 9102 for aerospace; QS-9000/ISO/TS16949, APQP for automotive; 21CFR, ISO 13485 for medical; plus, defense, heavy machinery, oil/gas/energy, and other multiple best-practice standards. Inspection results collected from the shop floor provide the source data used in these reports. If the QMS features a report designer, as some do, it allows the user to create custom report forms and templates to meet internal or external reporting requirements. Custom reports can include formulas, unique page layouts, and it also offers the capability to re-create your customers’ templates and branding. It’s one more way to solidify and nurture customer relationships with your company.
A truly comprehensive QMS also embraces and manages measurement gages and devices. While automatic ballooning and results reporting are often the tasks that generate the most interest in this kind of software, gage management is a close runner up because, again, it ties this vast function into one system. There are several good independent software programs dedicated solely to gage management. What’s compelling about having the centralized, all-in-one system strategy is that every gage used on a particular work order is identified and tracked. If there is a problem with a particular part lot down the road, the gage calibration data can be verified and used to ensure that the gages were within tolerance. It also provides the capability to find out if any other part lots might be affected that used those same gages. Additionally, when identifying the gages to be used on an upcoming job, staffers can readily determine in the system where those gages are currently located within the shop, who is using them, and whether a calibration alert might be popping up on any of the required gages. The quality technician or machine operator can then ensure that the calibration is done well in advance of the job start date.
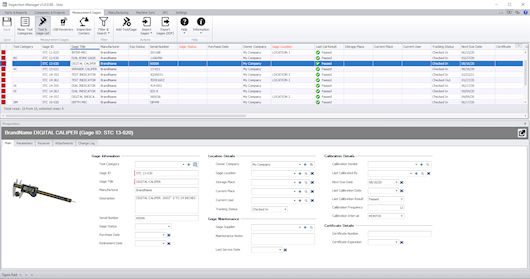
A truly comprehensive QMS also tracks and manages measurement gages and devices.
Data-driven manufacturing is here. The quality function is crucial in this endeavor, and while those of us in the quality department have always known this, that realization is becoming much more mainstream at the executive level. The question has been how best to align manufacturing operations with the quality function in the new digital, data-driven environment. We knew we needed some form of all-in-one system to remain competitive and profitable, however, the initial generations of these programs were lacking in one area or another. Those solutions are now available that are economical from a time and cost investment perspective and can be an answer to streamline and simplify data management. As an added bonus, the latest generations of comprehensive QMS technology gets all contributors in the manufacturing process on the same page with part dimensions, tolerances, and data reporting which maximizes process efficiency. An automated, centralized, integrated approach to inspection and measurement data management can significantly reduce errors, improve data security, and provide consistent product quality. Why? Because the source information – generated automatically right from the drawing or model, provides accurate and reliable data. That’s the solid, unshakable foundation necessary upon which to build the entire QMS structure and data-driven strategy in any manufacturing company.

High QA software automatically recognizes critical geometric dimension and tolerancing (GD&T) specifications from 2D prints, eliminating manual entry and reducing opportunity for error.
Details of any medical device’s manufacturing processes must be completely traceable so manufacturers can monitor product quality, make timely updates, and quickly recall defective items. Traceability extends from the original equipment manufacturer (OEM) to suppliers of individual components.
Should defects occur, cradle-to-grave tracking makes it possible to pinpoint exactly where changes in product quality initiated.
Medical device manufacturers face U.S. Food and Drug Administration (FDA) inspections and regulatory mandates as well as ISO requirements and risk management pressures. Quality shortcomings in an artificial joint or pacemaker can have lifelong or life-ending consequences, so tracing an error or omission to a single process is critical. Recently, some medical implant recalls have been tracked to failures of individual electronic components or to process errors such as inadequate part cleaning.

Software from High QA automatically extracts manufacturing and inspection requirements from a 2D drawing or PDF file, creating a ballooned inspection drawing and complete bill of dimensional characteristics stored in a single database.
Manufacturers can collect information almost instantly at multiple steps in a process, however such advances can generate massive amounts of data, and knowing exactly which are useful is a challenge. For OEMs, the key is determining which data are critical, translating them into useful information, and sharing appropriate information with suppliers.
Traditionally, supplier manufacturing engineers and quality assurance personnel decided which dimensions to inspect, how frequently to make inspections, and how to tabulate results. However, suppliers’ decisions don’t always match customer priorities.
An OEM can avoid data collection and reporting inconsistencies by presenting suppliers with a consistent set of standards, requiring the same information for inspection and reporting. Information should include drawings with part dimensions and establish a standard identification system for inspecting the characteristics of a part and recording the results. Manufacturers can achieve standardized identification of part requirements by annotating drawings with numbered callouts or balloons that point out individual part features. Balloon numbers correspond with numbers on a dimensional data form that lists dimensions, tolerances, and other requirements. Every supplier producing a certain part can use the same drawing balloon information to make the part, inspect it, and report results. Multiple suppliers can make the same part, delivering manufacturing and inspection data to the OEM in an identical format.

High QA’s inspection technology enables users to develop, control, and deploy inspection plans, related documents, requirements, and measurement instructions throughout an organization, providing an infrastructure to standardize and structure inspection data across an entire supply chain.
Automatically extracting geometric dimension and tolerancing (GD&T) information from the source part drawing eliminates interpretation and laborious manual tasks. Scanning the 2D drawing or PDF once for each part, via automatic ballooning and other quality requirements, assures dimensions are defined and numbered the same way on all suppliers’ drawings. A single source for dimensional information makes inspection results easily identifiable and able to be compiled for trend analysis showing dimensions in relation to specifications.
The OEM’s inspection plan can include instructions about inspection frequency – pointing out which part dimensions require 100% inspection and those that need less frequent inspection. Inspection information includes standardized serial numbers that are stored and recalled from a central database and can be searched and recovered quickly. Standardizing part feature identification and inspection plans can also help generate easily comparable supplier quotes.
Quality information management software gets all medical device manufacturing process contributors on the same page for part dimensions, tolerances, and data reporting – maximizing process efficiency. An automated, integrated approach to data management practically eliminates data-based errors and provides consistent product quality that protects the ultimate customer: the patient for whom the medical device can be a life-preserving resource.